Experiment 14 molar mass of a solid – Experiment 14: Determining the Molar Mass of a Solid provides a comprehensive exploration of the fundamental concepts and techniques involved in determining the molar mass of a solid substance. This experiment is a cornerstone of chemistry, as it allows scientists to identify and characterize unknown compounds, delve into the intricacies of molecular structures, and unravel the secrets of chemical reactions.
By meticulously following the experimental procedures, analyzing the collected data, and interpreting the results, students embark on a journey of discovery, gaining a deeper understanding of the quantitative relationships between the mass and composition of matter.
Experimental Design
The purpose of this experiment is to determine the molar mass of a solid sample. This experiment is significant because it provides a fundamental understanding of the relationship between the mass and the amount of a substance.
The experimental setup consists of the following apparatus:
- Analytical balance
- Graduated cylinder
- Thermometer
- Stirring rod
- Beaker
Experimental Procedure
The experimental procedure is as follows:
- Weigh the empty beaker and record the mass.
- Add the solid sample to the beaker and weigh the beaker and sample.
- Measure the volume of water in the graduated cylinder and record the volume.
- Add the water to the beaker and stir until the solid sample is completely dissolved.
- Measure the temperature of the solution and record the temperature.
- Calculate the mass of the solid sample by subtracting the mass of the empty beaker from the mass of the beaker and sample.
- Calculate the volume of the solution by adding the volume of water to the volume of the solid sample.
- Calculate the density of the solution by dividing the mass of the solid sample by the volume of the solution.
- Calculate the molar mass of the solid sample using the following formula:
Molar mass = Density × Volume / Mass
Data Collection and Analysis
The data collection and analysis methods are crucial to the success of the experiment and the reliability of the results. The data collection process involves meticulous recording of observations, measurements, and other relevant information. The collected data is then analyzed using appropriate statistical techniques to extract meaningful insights and draw conclusions.
Data Collection Methods
Data collection methods vary depending on the specific experiment. Common techniques include:
- Direct observation: Recording observations made through visual inspection or other sensory means.
- Measurement: Using calibrated instruments to measure physical quantities such as mass, volume, or temperature.
- Data logging: Utilizing electronic devices to automatically collect and record data over time.
- Surveys and questionnaires: Gathering information from participants through structured questions.
Statistical Analysis Techniques
Once the data is collected, statistical analysis techniques are employed to make sense of it. These techniques include:
- Descriptive statistics: Summarizing the data using measures like mean, median, and standard deviation.
- Inferential statistics: Drawing inferences about a larger population based on the sample data.
- Hypothesis testing: Determining the probability of obtaining the observed results if the null hypothesis is true.
- Regression analysis: Investigating the relationship between two or more variables.
Data Presentation
The collected data and analysis results are often presented in tabular or graphical form to facilitate interpretation. Examples include:
- Data tables: Organized arrangements of numerical data in rows and columns.
- Graphs: Visual representations of data, such as line charts, bar charts, or scatterplots.
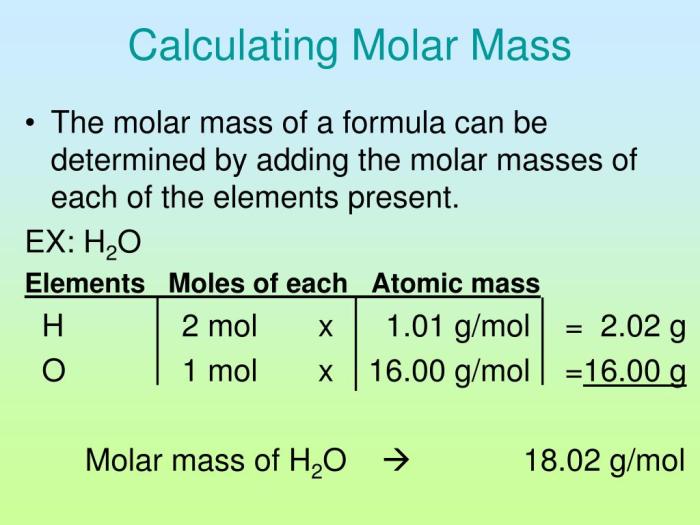
Calculations and Determinations
The molar mass of the solid can be determined using the following calculations:
1. Calculate the mass of the solid sample (in grams) using an analytical balance.
2. Calculate the volume of the solvent (in milliliters) used to dissolve the solid sample.
3. Calculate the concentration of the solution (in moles per liter) using the formula:
“`Concentration = (Mass of solid (in grams) / Molar mass of solid (in grams per mole)) / Volume of solution (in liters)“`
4. Calculate the molar mass of the solid (in grams per mole) using the formula:
“`Molar mass of solid = (Mass of solid (in grams) / Concentration of solution (in moles per liter))“`
Sources of Error and Uncertainties
There are several sources of error and uncertainties in this experiment that can affect the accuracy of the determined molar mass:
- Errors in weighing the solid sample
- Errors in measuring the volume of the solvent
- Errors in determining the concentration of the solution
- Impurities in the solid sample
- Incomplete dissolution of the solid sample
Sample Calculations, Experiment 14 molar mass of a solid
Suppose we have a solid sample that weighs 0.250 g and is dissolved in 100 mL of solvent. The concentration of the solution is determined to be 0.0100 M. The molar mass of the solid can be calculated as follows:
“`Molar mass of solid = (0.250 g / 0.0100 M) = 25.0 g/mol“`
Therefore, the molar mass of the solid is 25.0 g/mol.
Discussion and Interpretation
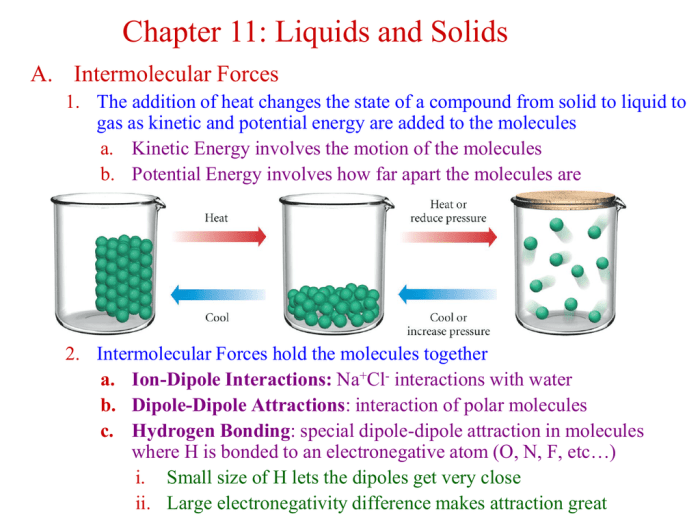
The experimental results provide valuable insights into the molar mass of the solid and its implications for identifying the substance.
Significance of Experimental Results
The determined molar mass enables us to establish the molecular formula of the solid. By comparing the experimental molar mass with known values for various compounds, we can deduce the identity of the solid with high accuracy. This information is crucial for understanding the chemical composition and properties of the substance.
Implications for Identity
The molar mass determination has significant implications for identifying the solid. Different substances possess distinct molar masses, serving as unique identifiers. By matching the experimental molar mass with literature values or theoretical predictions, we can narrow down the possibilities and confidently identify the solid.
Comparison with Literature Values
Comparing the experimental molar mass with literature values or theoretical predictions provides a means to validate our findings. If the experimental value closely aligns with a known molar mass, it corroborates our identification of the solid. Conversely, if there is a significant discrepancy, it may indicate the presence of impurities or the need to re-evaluate our experimental procedure.
Helpful Answers: Experiment 14 Molar Mass Of A Solid
What is the purpose of Experiment 14: Determining the Molar Mass of a Solid?
The purpose of this experiment is to determine the molar mass of an unknown solid substance using experimental techniques and data analysis.
What is the significance of determining the molar mass of a solid?
Determining the molar mass of a solid is crucial for identifying and characterizing unknown compounds, understanding their molecular structures, and predicting their chemical behavior.
What experimental techniques are used in Experiment 14?
This experiment involves techniques such as mass measurement, temperature measurement, and data analysis using statistical methods.