Which of the following reactions would result in increased entropy? This question delves into the fundamental concept of entropy in thermodynamics, a measure of disorder and randomness within a system. Understanding entropy is crucial for predicting the spontaneity and direction of chemical reactions, as well as its applications in various fields of chemistry and beyond.
Entropy is a key concept in thermodynamics, providing insights into the spontaneity and direction of chemical reactions. By examining different types of reactions and their impact on entropy, we can gain a deeper understanding of the underlying principles governing chemical processes.
1. Definition of Entropy
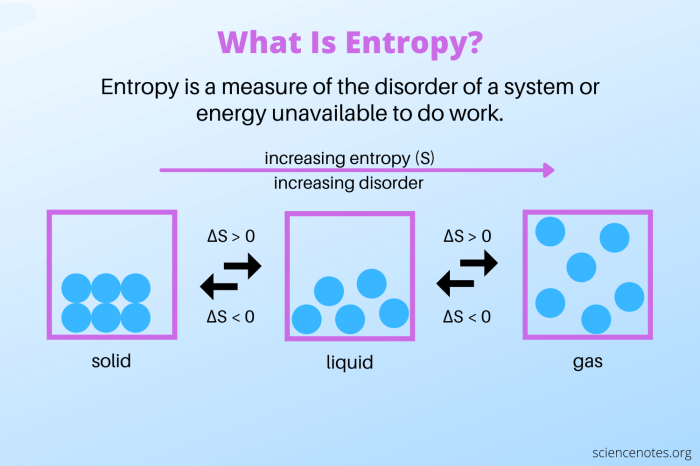
Entropy is a measure of the randomness or disorder in a system. In thermodynamics, entropy is defined as the logarithm of the number of possible microstates of a system.
A system with high entropy has many possible microstates, while a system with low entropy has few possible microstates. For example, a gas has higher entropy than a liquid, which has higher entropy than a solid.
2. Chemical Reactions and Entropy: Which Of The Following Reactions Would Result In Increased Entropy
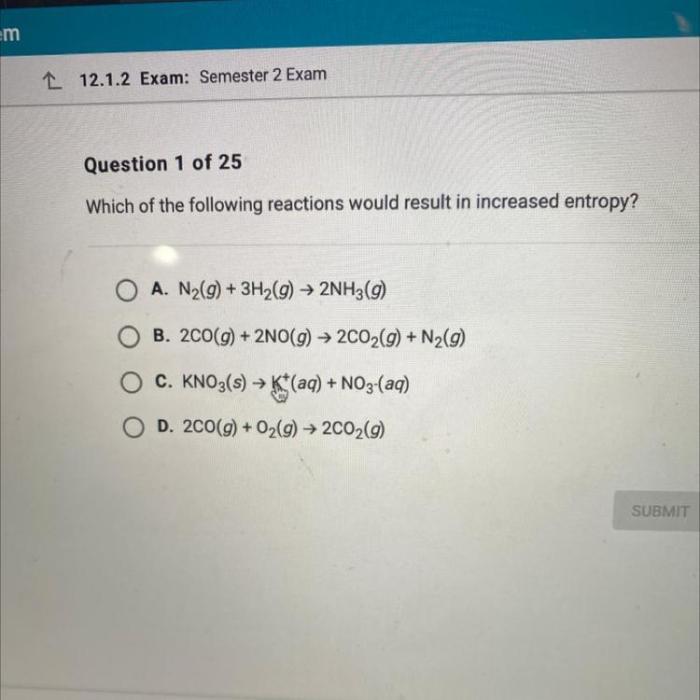
Chemical reactions can either increase or decrease entropy. Reactions that increase entropy are spontaneous, while reactions that decrease entropy are nonspontaneous.
The entropy change of a reaction can be predicted using the following equation:
“`ΔS = S products
Sreactants
“`
where ΔS is the entropy change, S productsis the entropy of the products, and S reactantsis the entropy of the reactants.
3. Types of Reactions that Increase Entropy
There are several types of reactions that typically result in increased entropy.
- Reactions that produce gases
- Reactions that produce more molecules
- Reactions that increase the temperature
- Reactions that decrease the pressure
4. Factors Affecting Entropy Changes
Several factors can affect entropy changes, including temperature, pressure, and concentration.
- Temperature:As temperature increases, the entropy of a system also increases.
- Pressure:As pressure increases, the entropy of a system decreases.
- Concentration:As concentration increases, the entropy of a system decreases.
These factors can be manipulated to control entropy changes.
5. Applications of Entropy in Chemistry
Entropy is used in various fields of chemistry, including thermodynamics, equilibrium, and kinetics.
- Thermodynamics:Entropy is used to calculate the spontaneity of reactions and to determine the equilibrium constant.
- Equilibrium:Entropy is used to determine the equilibrium concentrations of reactants and products.
- Kinetics:Entropy is used to determine the rate of reactions.
6. Entropy and Disorder

Entropy is closely related to disorder. A system with high entropy is more disordered than a system with low entropy.
Entropy can be used as a measure of disorder in a system. The more disordered a system is, the higher its entropy.
7. Entropy and the Second Law of Thermodynamics
Entropy is related to the Second Law of Thermodynamics, which states that the entropy of an isolated system always increases over time.
This means that all spontaneous processes are accompanied by an increase in entropy. Entropy can be used to predict the direction of spontaneous processes.
8. Entropy and Biological Systems
Entropy plays an important role in biological systems. Metabolism, for example, is a process that increases entropy.
Entropy can also be used to understand the evolution of life. The evolution of life can be seen as a process of increasing entropy.
Answers to Common Questions
What is entropy?
Entropy is a measure of disorder or randomness within a system. It quantifies the number of possible arrangements or microstates of a system.
How is entropy related to chemical reactions?
Chemical reactions can lead to changes in entropy. Reactions that result in an increase in disorder or randomness typically have a positive entropy change.
What factors affect entropy changes?
Temperature, pressure, and concentration can influence entropy changes. Increasing temperature and decreasing pressure generally lead to increased entropy.