Mercury metal is poured into a graduated cylinder, a seemingly simple task that requires meticulous attention to detail. This guide delves into the intricacies of measuring mercury metal using a graduated cylinder, exploring its unique properties, measurement techniques, and safety considerations.
The physical and chemical characteristics of mercury metal, its toxicity, and the accuracy and precision limitations of graduated cylinders are thoroughly examined.
Mercury Metal: Mercury Metal Is Poured Into A Graduated Cylinder
Mercury is a unique metal with distinct physical and chemical properties. It is the only metal that is liquid at room temperature and has a silvery-white appearance.
Properties of Mercury Metal
Mercury has several notable properties:
- Liquid State at Room Temperature:Mercury’s low melting point (-38.83 °C) and high boiling point (356.73 °C) result in its liquid state at room temperature.
- High Density:Mercury has a high density of 13.595 g/cm³, making it one of the densest elements.
- Low Vapor Pressure:Mercury has a relatively low vapor pressure, resulting in a low evaporation rate at room temperature.
- High Surface Tension:Mercury has a high surface tension, causing it to form spherical droplets and not wet surfaces.
- Toxicity:Mercury is highly toxic and can cause severe health effects if inhaled or ingested.
Graduated Cylinder Measurement, Mercury metal is poured into a graduated cylinder
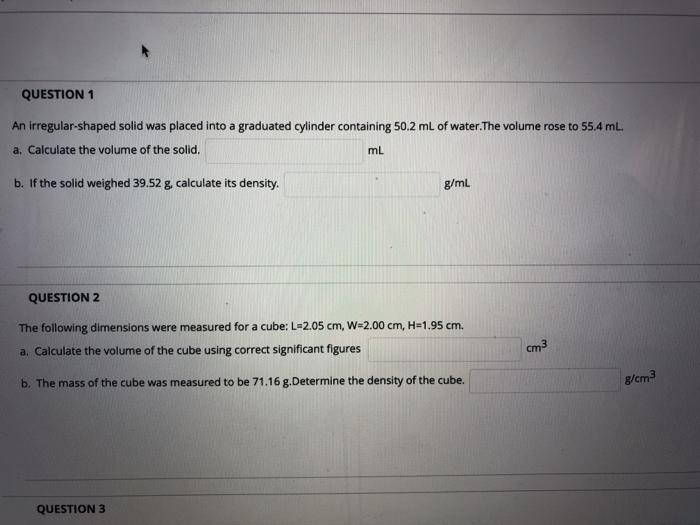
A graduated cylinder is a laboratory equipment used to measure the volume of liquids. It consists of a transparent cylindrical tube with a narrow spout and a calibrated scale marked along its length.
Graduated cylinders have varying degrees of accuracy and precision, depending on their size and the fineness of the scale markings.
Pouring Mercury into a Graduated Cylinder
To safely pour mercury into a graduated cylinder, follow these steps:
- Wear appropriate safety gear, including gloves and eye protection.
- Place the graduated cylinder on a stable, level surface.
- Hold the mercury container securely and slowly pour the mercury into the cylinder.
- Avoid spilling the mercury and keep the container steady.
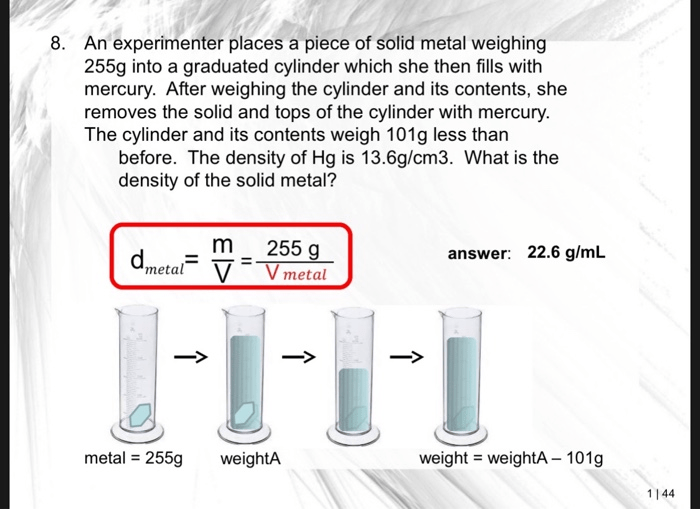
- Read the meniscus carefully to determine the volume of mercury poured.
Volume Determination
To determine the volume of mercury in the graduated cylinder, read the meniscus at eye level. The meniscus is the curved surface of the liquid. For accurate measurement, read the bottom of the meniscus for convex liquids (e.g., mercury) and the top of the meniscus for concave liquids (e.g.,
water).
Applications of Mercury Metal
Mercury has various industrial and scientific applications, including:
- Thermometers and Barometers:Mercury’s high thermal expansion and low vapor pressure make it suitable for use in thermometers and barometers.
- Electrical Switches:Mercury is used in tilt switches and mercury relays due to its high electrical conductivity.
- Amalgams:Mercury forms alloys with other metals, called amalgams, used in dental fillings and jewelry.
- Lighting:Mercury vapor lamps emit ultraviolet light, which is used in germicidal and tanning applications.
Safety Precautions
Working with mercury requires strict safety measures:
- Proper Handling:Handle mercury with care, using appropriate gloves and eye protection.
- Spill Prevention:Prevent spills by using spill trays and handling mercury containers carefully.
- Ventilation:Ensure adequate ventilation when working with mercury to avoid inhalation of vapors.
- Storage:Store mercury in sealed containers and dispose of it properly according to local regulations.
- Personal Hygiene:Wash hands thoroughly after handling mercury and avoid touching the face or eating.
FAQ Guide
What are the safety precautions when working with mercury metal?
Mercury metal is highly toxic and requires strict safety measures. Avoid skin contact, inhalation of vapors, and ingestion. Wear appropriate protective gear, work in well-ventilated areas, and follow proper disposal procedures.
How can I ensure accurate volume measurement when pouring mercury metal into a graduated cylinder?
Use a clean and calibrated graduated cylinder. Read the meniscus at eye level and estimate the volume to the nearest graduation mark. Repeat the measurement several times for increased accuracy.
What are the applications of mercury metal?
Mercury metal finds use in thermometers, barometers, and electrical switches. It is also employed in scientific research, dentistry, and the production of certain chemicals.