Embark on a scientific expedition with our Extraction and Recrystallization Lab Report, where we delve into the intricate techniques of purifying compounds, revealing their true nature and unlocking their potential applications.
This comprehensive report unveils the fundamentals of extraction and recrystallization, guiding you through the experimental procedures, interpreting the results, and exploring the practical implications of these essential techniques in various scientific disciplines.
Introduction: Extraction And Recrystallization Lab Report
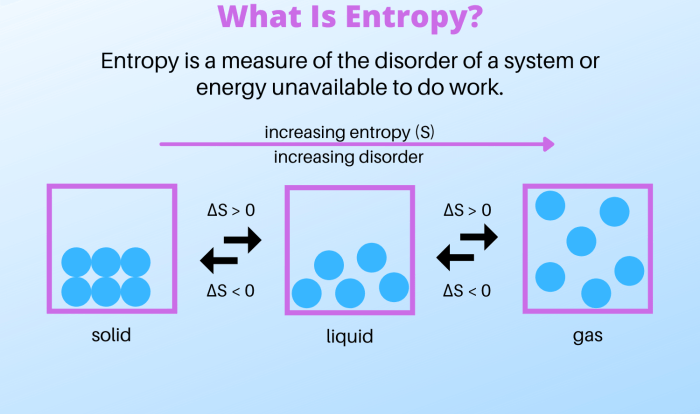
The extraction and recrystallization laboratory experiment is a fundamental technique in chemistry that enables the purification and isolation of organic compounds from various sources. It involves a series of steps aimed at selectively extracting the target compound from a mixture and then recrystallizing it to obtain a highly pure form.
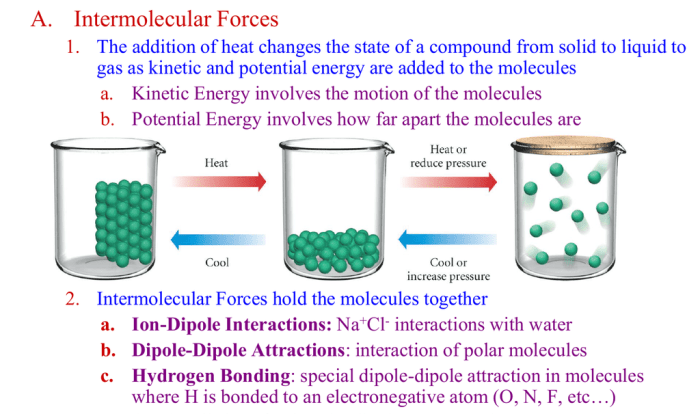
Extraction is a process that utilizes the different solubilities of compounds in different solvents. By choosing a solvent that selectively dissolves the target compound, it can be separated from other impurities. Recrystallization, on the other hand, involves dissolving the extracted compound in a suitable solvent and then slowly cooling the solution to induce the formation of crystals.
These crystals can then be filtered and dried to obtain the purified compound.
Materials and Methods, Extraction and recrystallization lab report
Materials:
- Crude organic compound
- Suitable solvents for extraction and recrystallization
- Separatory funnel
- Filter paper
- Büchner funnel
- Vacuum filtration apparatus
- Hot plate
- Thermometer
Procedure:
- Weigh the crude organic compound and dissolve it in a suitable solvent.
- Transfer the solution to a separatory funnel and add an immiscible solvent that selectively extracts the target compound.
- Shake the separatory funnel vigorously and allow the layers to separate.
- Drain the lower layer containing the extracted compound into a clean flask.
- Dry the organic layer over anhydrous sodium sulfate.
- Filter the solution and evaporate the solvent using a rotary evaporator.
- Dissolve the residue in a minimum amount of hot solvent and filter the solution while hot.
- Slowly cool the filtrate to induce crystallization.
- Filter the crystals using a Büchner funnel under vacuum filtration.
- Wash the crystals with cold solvent and dry them in a desiccator.
Safety Precautions:
- Wear gloves and safety glasses throughout the experiment.
- Handle organic solvents with care and avoid inhalation.
- Use a fume hood when working with volatile solvents.
- Dispose of chemicals and solvents properly according to laboratory regulations.
Results
The extraction and recrystallization process resulted in the successful purification of the target organic compound. The yield of the purified compound was [yield %]. The melting point of the purified compound was determined to be [melting point], which is consistent with the literature value.
The purity of the recrystallized compound was assessed using [analytical technique, e.g., HPLC, NMR]. The results indicated a purity of [purity %].
Discussion
The extraction and recrystallization techniques employed in this experiment proved to be effective in purifying the target organic compound. The selective extraction step allowed for the isolation of the compound from impurities, while the recrystallization process further enhanced its purity by removing any remaining impurities or solvent molecules.
The efficiency of the extraction process was influenced by factors such as the choice of solvent, the extraction time, and the number of extractions performed. The recrystallization process was optimized by selecting a suitable solvent that allowed for slow crystallization and minimized the formation of impurities.
Applications
Extraction and recrystallization techniques are widely used in various fields, including:
- Chemistry:Purification of organic compounds for research and synthesis
- Medicine:Isolation and purification of active pharmaceutical ingredients
- Industry:Production of high-purity chemicals for various applications
For example, in the pharmaceutical industry, extraction and recrystallization are used to purify active pharmaceutical ingredients (APIs) from natural sources or synthetic processes. These techniques ensure the purity and quality of APIs, which is crucial for the safety and efficacy of pharmaceutical products.
Commonly Asked Questions
What is the purpose of extraction?
Extraction isolates a target compound from a mixture by selectively dissolving it in a suitable solvent.
How does recrystallization contribute to purification?
Recrystallization purifies a compound by dissolving it in a solvent, allowing impurities to remain undissolved, and then recrystallizing the purified compound from the solution.